how to find core electrons|Core electron : Pilipinas We can see from the electron configuration of a carbon atom—1s 2 2s 2 2p 2 —that it has 4 valence electrons (2s 2 2p 2) and 2 core electrons (1s 2). You will see in . Klaviyo is a Customer CRM and Email Marketing Platform that allows Hilco Vision to collect sales and customer data in order to serve relevant marketing material and transactional communication. laravel_session. Default functionality of a website that has login sessions. It associates a user's browser with their login session on the server so .
PH0 · ⚗️ Identify Valence Electrons and Core Electrons
PH1 · What are Core Electrons?
PH2 · Valence electrons (video)
PH3 · How do you find core and valence electrons? + Example
PH4 · How do you find core and valence electrons? + Example
PH5 · Core electron
PH6 · CHEMISTRY 101: Valence and core electrons
PH7 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH8 · 3.4: Core and Valence Electrons
PH9 · 2.5: Electrons in atoms
PH10 · 1.9B: Valence and Core Electrons
Former Bayan Muna Rep. Teddy Casiño turned the tables on Duterte, reminding him about the alleged excessive force during his term. . Casino claimed people were even killed when cops served arrest warrants when Duterte was president. The former president earlier slammed the police use of ‘excessive force’ in its attempt to arrest .
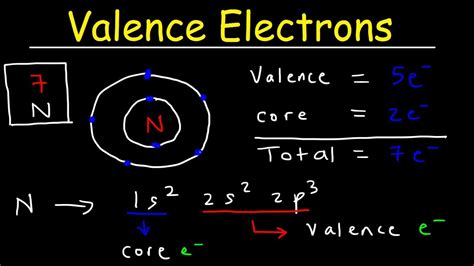
how to find core electrons*******Valence electrons occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy . We can see from the electron configuration of a carbon atom—1s 2 2s 2 2p 2 —that it has 4 valence electrons (2s 2 2p 2) and 2 core electrons (1s 2). You will see in . Write the electron configuration for phosphorus and identify the valence electrons and core electrons. Write the electron configuration for Ge by determining the total number of electrons. Learning Objective: Evaluate the number of valence electrons and core electrons from the electron configurations. Topics: core electrons, valence electrons.Quantization of electron energies, the arrangement of electrons in an atom, and the rules for determining the electron configuration of 1st twenty elements are described.The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell orbitals are called core . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as .
how to find core electronsCore electrons are the electrons in an atom that are not valence electrons and do not participate in chemical bonding. The nucleus and the core electrons of an atom form the .Core electron Core electrons are the electrons in an atom that are not valence electrons and do not participate in chemical bonding. The nucleus and the core electrons of an atom form the . How to Find Core Electrons? To find the number of core electrons in an atom, you can use the periodic table to identify the element and then subtract the .
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright .
2. Subtract the charge from the atomic number if the ion is positive. If the charge is positive, the ion has lost electrons. To . https://StudyForce.com https://Biology-Forums.com Ask questions here: https://Biology-Forums.com/index.php?board=33.0Follow us: Facebook: .
how to find core electrons Core electron Core electrons are the electrons that are found in the innermost electron shell of an atom. Core electrons are sometimes also referred to as “inner shell electrons.”. Core electrons are tightly bound to the nucleus and have the highest electron binding energy. The number of core electrons in an atom depends on the atomic number of the .Note that while we often refer to the Z eff of a valence electron, we can calculate the Z eff for any electron by taking into account only the number of core electrons that are shielding. For example, consider a 2 s electron of Cl. For Cl, Z = 17 and the electron configuration is 1 s2 2 s2 2 p6 3 s2 3 p5. The only electrons that will shield a 2 . 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.
The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell orbitals are called core electrons (Figure 6.28). Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that . Figure 8.4.1 8.4. 1: (a) The radius of an atom is defined as one-half the distance between the nuclei in a molecule consisting of two identical atoms joined by a covalent bond. The atomic radius for the halogens increases down the group as n increases. (b) Covalent radii of the elements are shown to scale.
in any case of any fraudulent, misdeclaration of the applicant in the use of these service, the prc may expel the applicant and prevent his/her further access to the prc's site, at any time for breaching the terms and conditions of this service or for violating the applicable laws.
how to find core electrons|Core electron